Research & Initiatives
The core-modified porphyrins resulting from the replacement of one or two pyrrole rings have received less attention inspite of their novel properties such as stabilization of metals in unusual oxidation states. Our group at IIT-Bombay, has been interested in synthesis of novel core-modified porphyrins to explore their potential for various applications in place of normal porphyrins (N4 core). We are working on different aspects of core-modified porphyrin chemistry and developed some new method(s) to synthesize new heteroporphyrin systems with desirable functional groups as well as some complicated heteroporphyrin based systems as outlined below:
1. Polycyclic Aromatic Hydrocarbon-/Heterocycle-Embedded Porphyrinoids
Polycyclic aromatic hydrocarbon (PAH)-/heterocycle-embedded porphyrinoids are porphyrinoids in which one or two pyrrole rings are replaced with polycyclic aromatic hydrocarbons/heterocycle moiety. These macrocycles possess the structural features of both polycyclic aromatic hydrocarbons/heterocycles and cyclic polypyrrolic porphyrinoids. The presence of a PAH moiety in the porphyrin core changes the electronic properties and PAH-embedded porphyrinoids may behave as nonaromatic/aromatic/antiaromatic systems.
2. Core-modified Porphyrinoids
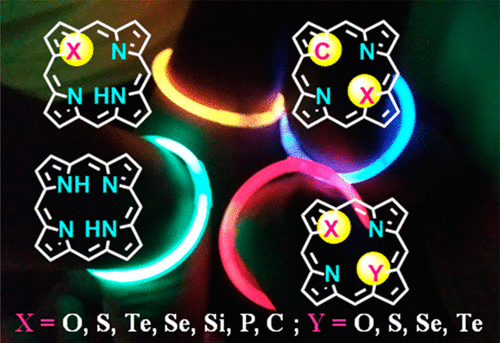
The heteroatom-containing porphyrin analogues or core-modified porphyrins that resulted from the replacement of one or two pyrrole rings with other five-membered heterocycles such as furan, thiophene, selenophene, tellurophene, indene, phosphole, and silole are highly promising macrocycles and exhibit quite different physicochemical properties compared to regular azaporphyrins. The properties of heteroporphyrins depend on the nature and number of different heterocycle(s) present in place of pyrrole ring(s). The heteroporphyrins provide unique and unprecedented coordination environments for metals. Unlike regular porphyrins, the monoheteroporphyrins are known to stabilize metals in unusual oxidation states such as Cu and Ni in +1 oxidation states. The diheteroporphyrins, which are neutral macrocycles without ionizable protons, also showed interesting coordination chemistry. Thus, significant progress has been made in last few decades on core-modified porphyrins in terms of their synthesis, their use in building multiporphyrin arrays for light-harvesting applications, their use as ligands to form interesting metal complexes, and also their use for several other studies. The synthetic methods available in the literature allow one to prepare mono- and diheteroporphyrins and their functionalized derivatives, which were used extensively to prepare several covalent and noncovalent heteroporphyrin-based multiporphyrin arrays. Our main focus is to develop synthetic strategies to develop the chemistry of different hetero analogues of porphyrin derivatives such as heterocorroles, heterochlorins, heterocarbaporphyrinoids, heteroatom-substituted confused porphyrins, heteroatom containing expanded porphyrinoids and very recently heteroatom containing norroles.
3. Triphyrrin
Recently, synthetic efforts to prepare novel modified porphyrinoid skeletons have been an important area of research due to their promising applications in various fields ranging from material chemistry to life science. The formation of [14]triphyrin(2.1.1) as relatively new congeners among the porphyrinoids seems quite astonishing because the porphyrin macrocyclic core itself is perturbed to afford a contracted structure retaining
aromaticity, however, exhibiting fundamentally different properties compared to other members in the porphyrin family. Although [14]triphyrin(2.1.1) chemistry has been mentioned in the literature, an effort which exclusively focussed on the development of [14]triphyrin(2.1.1) was not made. We are currently focusing on the chemistry of [14]triphyrin(2.1.1) including its synthesis, structure, coordination chemistry, photophysical and electrochemical properties. A fundamental insight in triphyrin chemistry has immense scope since the reactivity of the molecule is less explored other than using it as a ligand to prepare a few metal complexes and coupling reactions.
4. Synthesis of functionalized BODIPYs
Boron-dipyrromethenes/BF2-dipyrrins (BODIPYs) are highly fluorescent dyes with a wide range of applications in various fields because of their attractive photophysical properties. One of the salient features of BODIPYs is that the properties of the BODIPY can be fine-tuned at will by selectively introducing the substituent(s) at the desired location(s) of the BODIPY. The BODIPYs have several potential sites where the functional groups can be introduced and the functionalized BODIPYs can be used as building blocks to synthesize the desired BODIPY derivatives with interesting features. We are working on the synthesis of different types of functionalized BODIPYs where the functional group(s) were introduced directly at the meso-carbon, at the pyrrole carbons of the BODIPY core as well as at the boron centre. Further, their applications toward the synthesis of simple substituted BODIPYs to complex BODIPY-based systems are being explored in our laboratory.
5. Coordination chemistry of open-cyclic pyrrole-based ligands
The chemistry of open-chain pyrrole-based ligands has gained wide interest due to their natural occurrence as biliverdin. Metal complexes of pyrrole-based ligands are used in major applications that includes catalysis, sensors, MOFs, and light-harvesting materials. For example, Manderville and co-workers reported that Cu(II) complex of prodigiosin (three pyrrole ring containing ligand) takes part in DNA cleavage. Metal complexes of prodigiosin have been further used for antibacterial and anticancer studies. The metal complexes of tetrapyrrole or larger oligopyrrole ligands can act as templates for supramolecular assemblies since these oligopyrrolic ligands similar to oligopyridines, can form helical metal complexes which have inherent chirality. Depending on the kind of oligopyrrolic ligand and the type of metal employed, metal helicates have been reported as single-helix, double-helix, and triple-helix confirmations. We are currently exploring open cyclic pyrrole-containing ligands for coordination chemistry.